Your cart is currently empty!
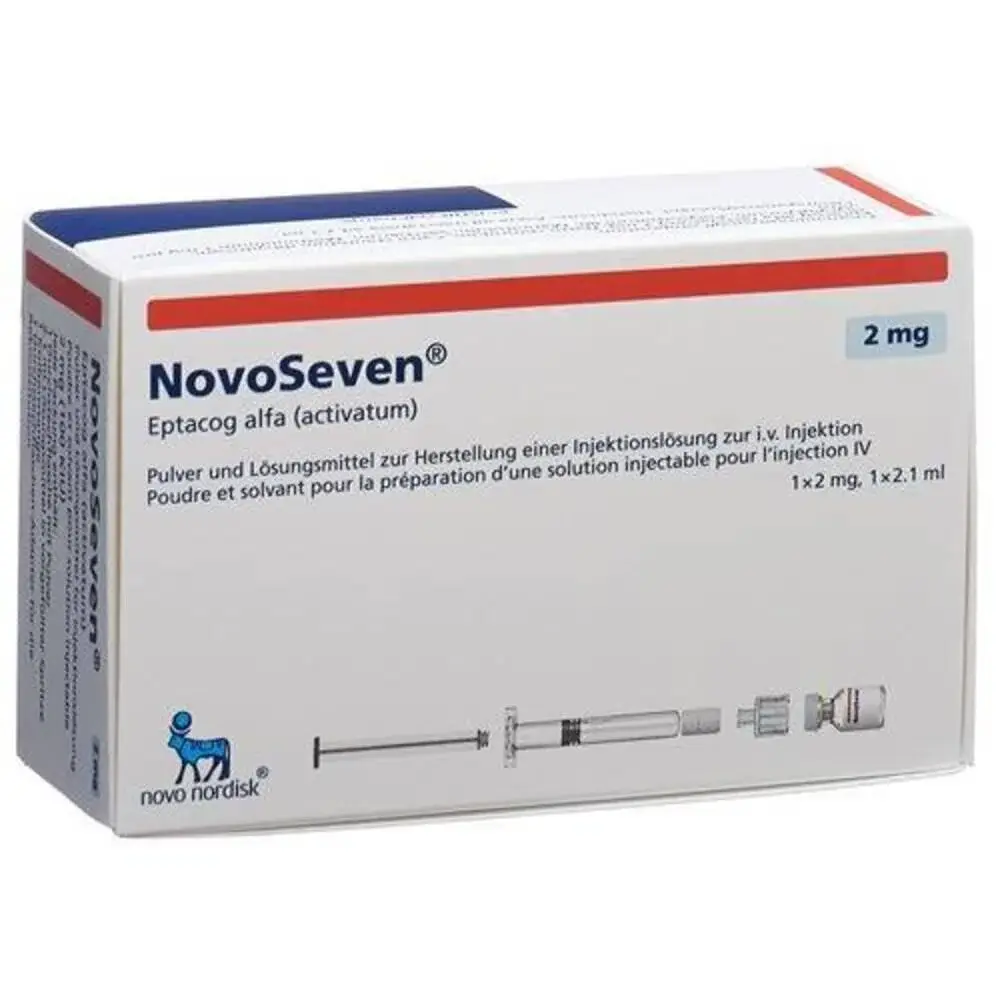
Novoseven (Factor Vii) Injection
Generic brands for Factor Vii Injection Available in India Brand Name Novoseven Generic Name Factor Vii Strength 2mg Manufacturer Novo Nordisk India Pvt Ltd
Description
Description
This page contains brief details about the drug factor vii, it’s indication, dosage & administaration, mechanism of action, related brands with strength, warnings and common side effects.
Date of Approval
Factor VII was initially approved by the United States Food and Drug Administration (FDA) on March 25, 1999.
Mechanism of Action of Factor Vii
Factor VII is a blood clotting protein that plays a pivotal role in coagulation. When tissue damage occurs, it interacts with tissue factor (TF) at the injury site, forming the FVIIa-TF complex. This complex acts as an enzyme, triggering a series of reactions that culminate in thrombin formation and the subsequent creation of a blood clot. This mechanism is essential for stopping bleeding after vascular injury.
Uses of Factor Vii
Factor VII is primarily used to treat bleeding disorders. Its key applications include managing bleeding episodes in hemophilia patients with inhibitors, addressing congenital Factor VII deficiency, controlling bleeding during surgery and trauma, managing acquired Factor VII deficiency associated with certain medical conditions, and occasionally, as part of treatment strategies for intracranial hemorrhage.
Factor Vii Dosage available
Factor VII concentrates are typically administered intravenously (IV) to individuals with bleeding disorders. A healthcare provider should determine the specific dosing and administration details based on the patient’s condition and needs.
We can ship to :
News/Updates
References
- Novo Nordisk A/S, Electronic medicines compendium (EMC), [Revised on 13 Jun 2023] [ Accessed on 2nd September 2023], https://www.medicines.org.uk/emc/files/pil.7927.pdf
- LFB S.A. Puteaux, US Food and Drug Administration, [Revised on Dec 2022] [ Accessed on 2nd September 2023],https://www.fda.gov/media/136610/download
- Katherine F Croom, Paul L McCormack; Recombinant factor VIIa (eptacog alfa): a review of its use in congenital hemophilia with inhibitors, acquired hemophilia, and other congenital bleeding disorders; BioDrugs; Published on 2008; Accessed on 02/09/2023; https://pubmed.ncbi.nlm.nih.gov/18345709/