Your cart is currently empty!
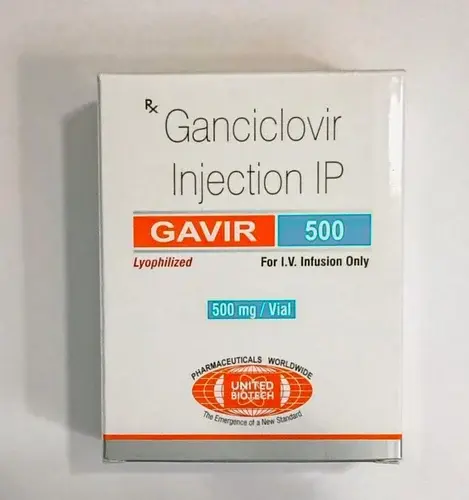
Gavir (Ganciclovir) Injection
Generic brands for Ganciclovir Injection Available in India Brand Name Gavir Generic Name Ganciclovir Strength 250mg, 500mg Manufacturer United Biotech Pvt Ltd
Description
Description
This page contains brief details about the drug ganciclovir, it’s indication, dosage & administaration, mechanism of action, related brands with strength, warnings and common side effects.
Date of Approval
Ganciclovir contains the active ingredient Ganciclovir. This medicine is used to treat and prevent cytomegalovirus (CMV) infections. It is used to treat CMV infections in AIDS patients and prevent CMV infections in people who received an organ transplant from someone infected with this virus. Cytomegalovirus can affect anyone but is mainly seen in people with a weak immune system. It can affect any part of the body, including the retina at the back of the eye, causing eye problems. Ganciclovir received FDA approval on June 18, 1989, for treating cytomegalovirus (CMV) retinitis in immunocompromised individuals, including those with HIV.
Mechanism of Action of Ganciclovir
Ganciclovir is an antiviral medication that exerts its effect by inhibiting the replication of cytomegalovirus (CMV) in the body. By suppressing CMV viral activity, this medication helps to control CMV infections. It reduces the risk of disease progression, including vision loss.
Uses of Ganciclovir
Ganciclovir is used to treat and prevent cytomegalovirus (CMV) infection.
Ganciclovir Dosage available
Ganciclovir can be consumed orally as capsules or administered intravenously (IV). The capsules are typically taken with water, with or without food, following the prescribed dosage and frequency for oral consumption. When administered intravenously, a healthcare professional delivers it via injection. The method of consumption depends on the specific formulation prescribed and should be followed as directed by a healthcare professional.
We can ship to :
News/Updates
References
- KD Tripathi, Essentials of Medical Pharmacology, Antiviral drugs, 7th edition, 2013, 808.
- Goodman & Gilman’s, The Pharmacological Basis of Therapeutics, Antiviral agents (Non-retroviral), 12th edition, 2011, 1615.
- Gilead Sciences Ltd, Electronic medicines compendium (EMC), [ Revised on Jan 2021] [ Accessed on 18th May 2023], https://www.medicines.org.uk/emc/files/pil.2314.pdf
- Gilead Sciences, US Food, and Drug Administration, [ Revised on Feb 2020] [ Accessed on 18th May 2023], https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208464s008lbl.pdf